
Earlier this month, the United States Court of Appeals for the Federal Circuit issued an opinion in the case of Novartis AG et al., v. Ezra Ventures LLC, case number 2017-2284, again finding that the judicially created, non-statutory, obviousness-type double patenting doctrine may not be used to invalidate or cut-short additional patent term conferred under the patent term extension (PTE) provisions of 35 U.S.C. § 156.
The court previously addressed a similar question in the case of Merck & Co. v. Hi-Tech Pharmacal Co., 482 F.3d 1317 (Fed. Cir. 2007), in which it held that obviousness-type double patenting does not invalidate an otherwise validly obtained PTE under § 156. In this recent opinion, the court addressed whether a PTE granted to a first patent under § 156 is improper if it “effectively” extends the patent term of a second patent.
This case concerns two patents owned by Novartis AG (Novartis) – U.S. Patent No.’s 5,604,229 and 6,004,565. The ’229 patent covers a large number of compounds including fingolimod, and the’565 patent covers a method of administering fingolimod. Novartis filed an infringement suit against Ezra Ventures LLC (Ezra) asserting infringement of the ’229 patent. Ezra filed a motion for judgment on the pleadings arguing that the ’229 patent should be ruled invalid, or otherwise terminally disclaimed as to any patent term that exceeded the expiration date of the ’565 patent, on the grounds of non-statutory, obviousness-type double patenting.
The patent application that would mature into the ‘229 patent originated from the national stage filing of a PCT application filed on October 18, 1993, with a national stage filing date of June 17, 1994. The ’229 patent issued on February 18, 1997. As the ’229 patent was filed before the effective date of the Uruguay Round Agreements Act of 1994 (URAA), the patent was entitled to a term that was the better of 17 years from issuance or 20 years from the earliest effective U.S. filing date. Based on the 17-year option, the ’229 patent would expire on February 18, 2014. However, pursuant to 35 U.S.C. § 156, Novartis secured a five-year patent term extension (PTE) for the ’229 patent based on delays in acquiring regulatory approval from the Food and Drug Administration. As a result of the PTE, the ’229 patent had an extended term that would expire on February 18, 2019.
The patent application that would mature into the ’565 patent was filed on September 23, 1997, and issued on December 21, 1999. Based on the standard 20-year term in effect at that time, the ’565 patent would expire on September 23, 2017.
The following timeline illustrates the relevant dates for the ’229 and ’565 patents:
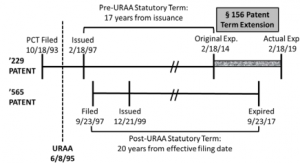
By arguing obviousness-type double patenting between the ’229 and ’565 patents, Ezra sought to limit the maximum term of the ’229 patent to expire concurrently with the ’565 patent on September 23, 2017. Though recognizing the prior precedent set by Merck, Ezra argued that the PTE granted to the ’229 patent was improper in this instance as it violates the language of § 156(c)(4), which states that “in no event shall more than one patent be extended under subsection (e)(1) for the same regulatory review period for any product.” Ezra argued that extension of the ’229 patent term also “effectively” extends the ’565 patent term because the ’229 patent covers a compound necessary to practice the methods claimed by the ’565 patent.
The court found Ezra’s argument unpersuasive as it would require reading the word “effectively” into the statutory language of § 1.56(c)(4). Specifically, the court reasoned that the intention of § 1.56(c)(4) is to limit a legally conferred PTE, not an “effective” or “de facto” PTE, to one patent selected by the patent owner. In this instance, the court found Novartis to have properly complied with the language of § 1.56(c)(4) by invoking § 156 to extend the term of only the ’229 patent, with a certificate of extension recorded in the official file of the patent and considered as part of the original patent. The court held that the fact the method of the expired ’565 patent cannot be practiced during the ’229 patent’s extended term is a permissible consequence of the legal status conferred upon the ’229 patent by § 156.
Upon finding the PTE granted to the ’229 patent proper, the court then followed the precedent of Merck in refusing to invalidate the extended term of the ’229 patent based on obviousness-type double patenting.

